Todays battles are going to be:
MRCP revision battle 20.1: MEN
MRCP revision battle 20.2: Folate
MRCP revision battle 20.3: Subacute combined degeneration of the spinal cord
MRCP revision battle 20.4: Vitamin B12
MRCP revision battle 20.5: Pernicious anaemia
MRCP revision battle 20.6: Churg Strauss
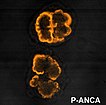
MRCP revision battle 20.7: ANCA
MRCP revision battle 20.1: MEN
As any of my friends will tell you MEN have frequently been a problem in my life and the condition MEN (=multiple endocrine neoplasia) has been no less troublesome. Yes, I get that they are genetic syndromes in which there are functioning hormone-producing tumours in multiple organs. My problem lies in managing to associate the different patterns to the different classes of MEN, which MRCP seems to require you to do rather a lot.
Firstly, 2 key facts to grasp:
- all MEN can be inherited or sporadic; if inherited they are autosomal dominant
- all are associated with hypercalcaemia, especially MEN 1
So, to try and learn the subtypes... I've settled on learning "Para pits against the pan men, for which Phaeo gives a medal to the para" (= parathyroid, pituitary, pancreas (men gene), phaechromocytoma, medullary thyroid, parathyroid)
Which, in more conventional terms:
MEN1
- parathyroid (95%), pituitary (70%) and pancreas (50%)
- caused by mutation of Menin gene (a tumour supressor) on chromosome 10
MEN 2a
- phaechromocytoma (95%), medullary thyroid cancer (70%) and parathyroid (60%)
- ret gene on chromosome 11
MEN 2b
- MEN 2a but without the parathyroid tumours and with a Marfarnoid appearence and mucosal neuromas
- also caused by the ret gene on chromosome 11
Note that the medullary thyroid cancer in MEN is in general less aggressive than the sporadic forms but prophylactic thyroidectomy should still be considered.
As a random aside it might be worth learning that in MEN the mutation in the ret gene is activating, whereas in Hirschsprung's disease, which is also caused by a ret gene mutation, the mutation is inactivating.
So, have you got Para pits against the pan men, for which phaeo gives a medul to the para... not ideal but its the best I've come up with....
Lets move on for a brief encounter with folate....